Chemogenetic tools to observe cell biology at various scale in space and time
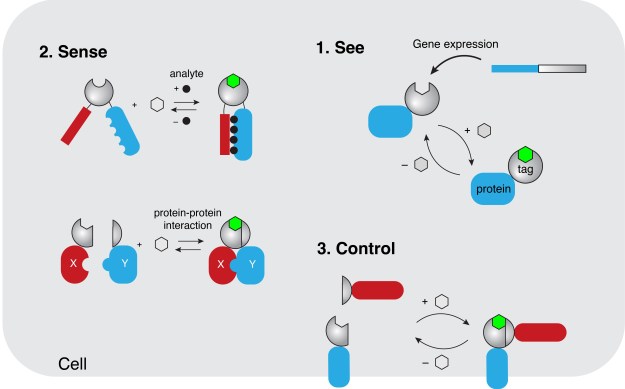
Fluorescent reporters and sensors play a central role in biological and medical research. Targeted to specific biomolecules or cells, they allow the visualization of the mechanisms that govern cells and organisms in real time. Recently, chemogenetic reporters composed of organic chromophores interacting with a protein moiety have challenged the hegemony of fluorescent proteins classically used in Cell Biology. Combining the advantage of synthetic fluorophores with the targeting selectivity of genetically encoded systems, these chemogenetic reporters open new perspectives for the study of cellular processes.

Our team has recently introduced a new class of chemogenetic fluorescent reporters, called FASTs (fluorescence-activating and absorption-shifting tags), which allow the visualization of gene expression and protein localization in living cells and organisms (PNAS 2016, Chem. Sci. 2017, Bioconjug. Chem. 2018, Angew. Chem. 2020, Nat. Chem. Biol. 2021, Nat. Commun. 2021, Acc. Chem. Res. 2022). Engineered using a concerted strategy of molecular engineering and directed protein evolution, FASTs are small protein tags that bind and stabilize the fluorescent state of fluorogenic chromophores. Dark when free in solution or cells, these so-called fluorogens allow the imaging of FAST-tagged proteins with very high contrast without the need for washing. FAST and its variants have proved to be a useful tool that is compatible with multiple microscopy modalities and model organisms. It excels in applications in oxygen-poor environments or in situations where the lack of delay in the formation of a fluorescent complex allows the detection of rapid biological events. Recently, we expanded our toolbox with near-infrared chemogenetic fluorescent reporters (Nat. Commun. 2025a) for advanced in vivo imaging and fluorescence lifetime-modulating tags for highly multiplexed imaging in cells and organisms (Advanced Science 2024). The modular nature of these reporters allowed us furthermore to design biosensors for the detection of key metabolites (ACS Chem. Biol 2018, ACS Sensors 2023). Finally, bisection of FASTs into two complementary fragments enabled the design of split fluorescent reporters with rapid and reversible complementation for the imaging of dynamic protein-protein interactions (Nat. Commun. 2019, ACS Chem. Biol. 2024, Nat. Commun. 2025b, ChemBioChem 2025). This latter technology holds great potential for developing screening strategies to identify stabilizers or disruptors of protein-protein interactions, which could have therapeutic applications.
Our current research interests focus on:
- innovative chemogenetic optical reporters and sensors for imaging cell biology at various scale in space and time using advanced microscopy.
- generic tools to image endogenous proteins to systematically study protein function in a native cellular background.
- optical sensors of protein-protein, organelle-organelle, and cell-cell interactions.
Chemogenetic tools to control cellular functions in cell biology and biomedicine
The specificity of cellular functions results from the spatial and temporal organization of functionally interacting proteins. Various strategies are used by cells to achieve specificity including protein compartmentalization in organelles, protein colocalization on membranes, or assembly of protein complexes mediated by specific scaffolds. Such spatial organization enables to increase effective molarity in biochemical processes and is essential for key cellular processes such as gene regulation, protein transport, organelle transport and positioning, signal transduction, metabolism, immune response or cell-cell communications. To study and understand the role of the spatiotemporal organization of proteins in these processes, we recently introduced CATCHFIRE (Chemically Assisted Tethering of CHimera by Fluorogenic Induced Recognition), a chemogenetic tool enabling to control the physical proximity of proteins and quantify this proximity (Nat. Methods 2023). This tool relies on the genetic fusion of two small dimerization domains that can interact together in presence of a fluorogenic inducer of dimerization that fluoresces upon formation of the ternary assembly, allowing real-time monitoring of chemically induced proximity. This technology allows the fine control of biological processes through chemically induced proximity of proteins.
Our current research interests focus on:
- Chemically induced proximity tools to manipulate and control biochemical processes for cell biology applications.
- Small-molecule-based control modules for applications in cell biology and cell therapy.